If yes, I must ask what is wrong with you. (YOU VERY SAD PERSON)
Anyhow, now I've asked the question, I feel obliged to give an answer.
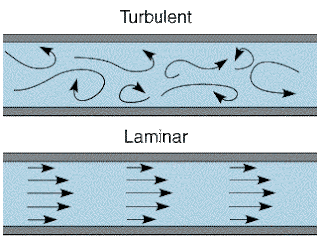
What it boils down to is the two different types of airflow; laminar and turbulent. Laminar flow is characterised by a system of orderly layers, with no eddies or irregular fluctuations. In simpler terms, the flow lines do not cross.
Laminar flow past a ball is bad; it forces a larger separation in the air flows, causing greater drag, so that the ball will not travel very well. However, the balls dimples cause the flow lines to cross, and creates a turbulent stream behind the ball, lessening drag.
Really, all I needed to say was that golf balls with dimples travel further... But that would have been no fun.
Anyhow, now I've asked the question, I feel obliged to give an answer.
What it boils down to is the two different types of airflow; laminar and turbulent. Laminar flow is characterised by a system of orderly layers, with no eddies or irregular fluctuations. In simpler terms, the flow lines do not cross.
Really, all I needed to say was that golf balls with dimples travel further... But that would have been no fun.